Definition of condensation reaction:
A condensation reaction is any kind of chemical reaction where two small molecules combine to form a new larger molecule. Firstly and most importantly this occurs in the presence of a catalyst and eliminates water or any other simple molecule. When the reaction destroys water molecules, we call this reaction the dehydration synthesis. However, other molecules such as acidic acid, ethanol, ammonia, etc., can also be eliminated. Aldehydes, ketones, esters, and alkynes are compounds that combine with each other or to themselves. However, amines are the only compound that connects with other organic compounds but not among themselves.
Complex metals, acids, cyanide ions, and bases are used as catalysts in this reaction. However, we consider aldehydes as aldols who have α-hydrogen along with the dilute base to provide β–hydroxy aldehydes. These aldols can participate in aldol condensation. Accordingly, when two different carbonyl compounds participate in aldol condensation, it becomes crossed aldol condensation.
Mechanisms of condensation reaction
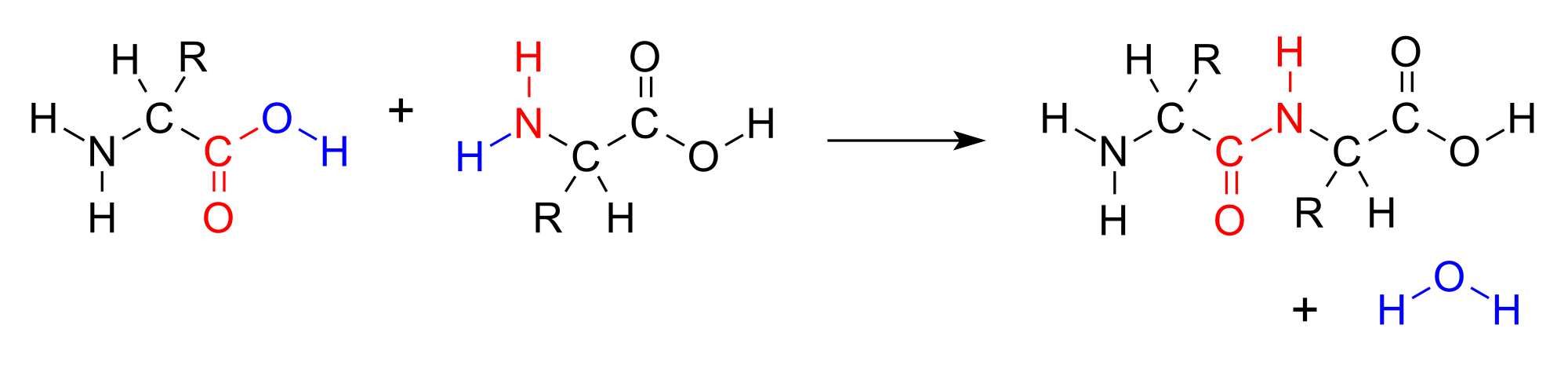
A condensation reaction is a chemical reaction of two different or identical compounds combined to form a single and large mixture of compounds. Sometimes the reaction eliminates water as a smaller molecule. However, there is an amine group on one side of the molecule and a carboxylic acid group on the other side in amino acids. Both of the amine and carboxylic acid groups perform as functional groups.
However, when these two amino acids combine through a condensation reaction, a bond appears between the two functional groups. The bond appears between alpha-amino acid’s amine nitrogen and the beta-amino acid’s carboxyl carbon. Hence, a water molecule leaves as a second product.
Other names of condensation reaction
Every condensation reaction has the same mechanism where two same molecules or two different molecules combine to form a larger molecule. However, this molecule can be aldehydes, ketones, esters, and alkynes.
However, based on the second product or the compound used in the reaction, we can differentiate the condensation reaction or give them different names.
Usually, the compounds used in this reaction are called aldols. So, the reaction gets the name aldol condensation.
When two same aldols/compounds perform in a condensation reaction, it becomes a self-condensation reaction.
Again, on the other hand, when two different aldol/compounds perform in a condensation reaction, it becomes a crossed aldol condensation reaction.
Consequently, most of the condensation reaction often reduces water molecules as a second product. This is why we call this dehydration synthesis reaction too.
Example of self-condensation reaction:
Acetone is that type of compound/aldol that can perform a self-condense and produce mesityl oxide.
For example, here we have two molecules of acetone in the presence of resin that can exchange ions and produce mesityl oxide.
Reaction:
2 CH3COCH3 → (CH3)2C=CH(CO)CH3 + H2O
Example of crossed aldol condensation:
Crossed aldol condensation only occurs when both of the aldehydes have alpha hydrogens and can act as carbanion. For example, we are taking two aldehydes that are acetaldehyde and benzaldehyde. Crossed aldol condensation of these two compounds will take place in the following steps:
First step
In this first step, a proton is removed from the alpha-carbon of acetaldehyde. However, the hydroxide ion of alkali removes the proton to give a carbanion.
Second step
In this step, the carbanion creates a nucleophilic state and attaches with the carbonyl carbon of benzaldehyde to make an alkoxide ion.
Third step
Finally, in the 3rd step, the alkoxide ion gets a proton from the water molecule to form beta-hydroxy aldehyde.
Aldol condensation
Application of aldol condensation:
- Gluconeogenesis and phosphate synthesis.
- Some companies use this reaction as a mediator in the process of making perfumes such as alpha-amyl cinnamic aldehyde, pseudo methyl ionone, cinnamic aldehydes, and alkyl-a-methyildihydro, etc.
- However, this reaction is also useful in the pharmaceutical industry.
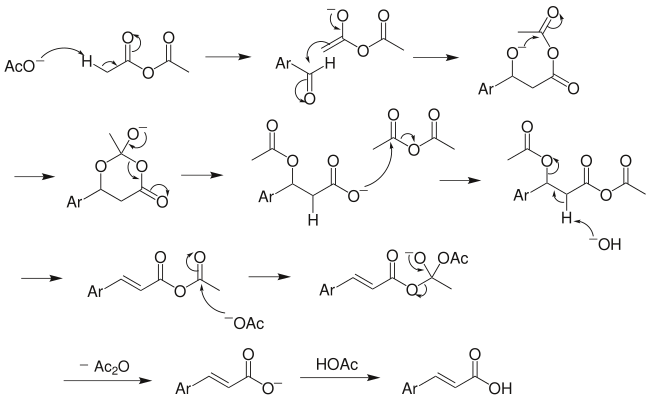
Perkin reaction
In the presence of an alkyl salt of the acid, the perkin reaction occurs. Firstly and most importantly, it is a reaction between an aromatic aldehyde and an acid anhydride. This reaction finally produces alpha, beta-unsaturated aromatic acid. However, in this reaction, the alkyl salt of the acid works as a base of the catalyst.
This reaction is named after William Henry Perkin.
Reaction:
Aromatic aldehyde
+
Aliphatic Acid anhydride
+
Alkali salt of the acid
↓
Cinnamic acid derivatives
Mechanism:
Henry nitroaldol reaction
It is an organic chemical reaction. In this reaction, the nitroalkanes react with alpha hydrogen and an aldehyde or ketone to produce beta-nitro alcohol. However, a base catalyst is required to complete the reaction.
Mechanism:
Firstly, and most importantly, the reaction begins with the deprotonation of the nitroalkane present on the α-carbon position and forms a nitronate. However, most nitroalkanes have a pKa of nearly 17. Although, because of the deprotonated carbon at the end and the oxy-anions of the nitro group, this structure is nucleophilic. As a result, the carbon attacks the carbonyl compound. Therefore, the conjugate acid of the originally deprotonated base protonated β-nitro alkoxide. This produces the β-nitro alcohol as a product.

Stobbe reaction
It is the reaction of aldehyde or ketone with the succinic ester. However, we need the presence of a basic catalyst to complete the reaction.
Mechanism:
Application:
- This reaction helps in the production of naphthol and indanone derivatives.
- Tetralone derivatives.
- Similarly, Phenanthrene derivatives.
Esterification condensation reaction
In this condensation reaction, two molecules of alcohol and carboxylic acid finally convert into larger molecules, esters. However, the name of this procedure is esterification. However, water molecules are eliminated from the reaction as a second product.
Mechanism:
Benzoin condensation
In this reaction, we get to see the involvement of two molecules of aldehydes. It occurs between aromatic aldehydes or glyoxals. This coupling reaction produces alpha-hydroxy ketones as the final product.
Mechanism:
Applications:
- It is a useful reaction to synthesize heterocyclic compounds.
- Accordingly, this reaction is also useful in the expansion of the aliphatic form of aldehyde.
- In the same way, this reaction helps the production of polymers. It is also useful in the condensation of new monomers.
Acyloin condensation
This is a coupling reaction where two carboxylic esters convert into an alpha-hydroxy ketone in the presence of metallic sodium.
Mechanism:
Applications:
- This reaction helps in the preparation of cyclic acyloin. It converts the long-chain dicarboxylic ester into a large ring compound. This process doesn’t use any dilution techniques. Hence, the best use of this reaction is to close rings of ten members or more.
- Catenane is a complex compound composed of interlocking rings.
Pechman condensation
The other name of this reaction is coumarin synthesis.
It is a synthesis of coumarins that is produced from phenol and a carboxylic acid or ester. In addition, the Ester in this reaction contains a β-carbonyl group. However, acidic conditions are required to perform this reaction. Using highly activated phenols, we can perform this reaction under much milder conditions.
Mechanism:
Condensation polymers
It is any kind of reaction that produces polymers from the condensation of two smaller molecules. In the same way, the reaction eliminates water molecules as byproducts.
As an example, Do-amides and carboxylic acid react with each other and produce the most common condensation polyamide, Nylon.
Mechanism:
Some frequently asked questions about condensation reactions.
Q. What happens in the condensation reaction?
Ans. In the condensation reaction, two smaller molecules react and combine with each other to produce a new larger molecule. In other words, the combination of two molecules is called a condensation reaction.
Q. What is the Cyclocondensation reaction?
Ans. It is basically the condensation reaction of heterocycle synthesis.
Q. How do you identify a condensation reaction?
Ans. In the condensation reaction, two smaller molecules combine and produce a new larger molecule. However, the reaction also causes the elimination of water molecules as a second product.
Q. Is condensation reversible?
Ans. Yes, physical changes of the compound that involve a change of state are reversible.
Q. Where do condensation reactions occur?
Ans. A condensation reaction occurs when two smaller molecules combine with each other and eliminate water as a byproduct to produce a new larger molecule.
Q. Is Sweating an example of condensation?
Ans. No, we can consider vapour transformation to water (condensed state) as a condensation reaction. But the opposite doesn’t define condensation reaction.